Lead-acid batteries, as a well-established energy storage technology, are widely used in data centers, telecommunications, and other fields. During practical use, overcharging and overdischarging pose significant threats to battery performance and operational safety. A Battery Management System (BMS) for lead-acid batteries plays a critical role by precisely monitoring and effectively preventing such issues.
Gassing
Overcharging causes water electrolysis inside the battery, producing significant amounts of hydrogen and oxygen. The accumulation of these gases increases internal pressure, especially in sealed batteries like VRLA (Valve Regulated Lead-Acid) batteries. If the gas pressure is not promptly released, it can lead to battery casing bulging, deformation, or even explosion.
Grid Corrosion
During overcharging, the lead plates within the batteries undergo oxidative corrosion, forming lead oxides, which may weaken the mechanical strength and conductivity of the plates, reducing the battery’s capacity and increasing the risk of active material shedding. The detached material may be deposited at the battery’s bottom, further increasing internal resistance and reducing efficiency.
Heat Generation (Thermal Runaway)
Both overcharging and overdischarging generate excessive heat within the battery. When the temperature exceeds safe limits, the evaporation rate of the electrolyte accelerates, and the chemical reactions on the plates may become uncontrollable, leading to thermal runaway. Although thermal runaway in lead-acid batteries rarely results in fire, it can cause acute battery failure, severely shortening the battery’s lifespan.
Battery Bulging
Excessive gas and heat accumulation during overcharging or overdischarging significantly increase internal pressure, causing the battery to bulge. This not only damages the battery's exterior but also compromises internal structures, such as separator deformation or rupture, leading to rapid capacity degradation and, in severe cases, complete failure.
Sulfation
Overdischarging leads to the formation of large lead sulfate crystals on the battery plates (sulfation). Such crystals, covering the plate surface, hinder chemical reactions between the electrolyte and the plates during charge and discharge cycles. Sulfation can significantly reduce battery capacity and performance, and potentially lead to irreversible failure.
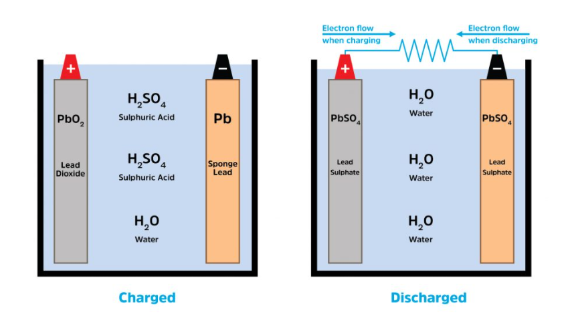
Charging and discharging of lead acid batteries
The adverse effects of overcharging and overdischarging severely impact the safety and lifespan of lead-acid batteries. To address these issues, modern lead-acid battery systems incorporate Battery Management Systems (BMS). A BMS continuously monitors key parameters such as battery voltage, current, and temperature. When the battery voltage approaches the critical thresholds of overcharging or overdischarging, the BMS promptly alerts users to take necessary actions.
Gerchamp G-TH BMS, equipped with the G-TC module, offers 24/7 real-time monitoring of critical metrics like charge and discharge currents. By integrating advanced BMS technologies of Gerchamp, lead-acid batteries can achieve longer lifespans, higher safety standards, and improved efficiency in demanding applications.